| Surfactant molecular aggregates in green solvents |
| From: PublishDate:2012-04-18 Hits: |
In recent years, supercritical CO2 (SC CO2) and ionic liquids (ILs), considered as unconventional and green solvents, have received much attention. The SC CO2 solvent is readily available, inexpensive, nontoxic, and nonflammable. More importantly, its physical and chemical properties can be easily adjusted by varying the operating pressure and temperature. ILs are also an interesting class of tunable solvents with essentially zero vapor pressure, wide electrochemical window, nonflammability, high thermal stability, and wide liquid range. The researchers from the Institute of Chemistry, Chinese Academy of Sciences have developed a series of novel surfactant molecular aggregates by using SC CO2 and ILs.
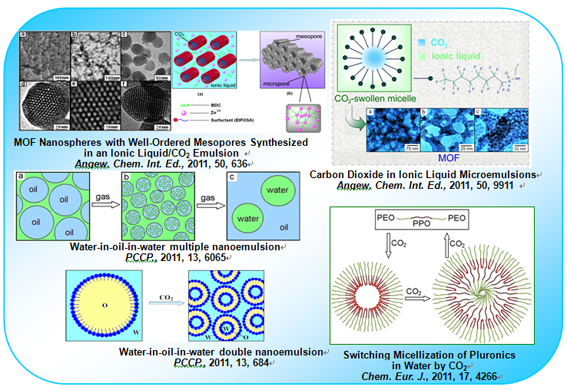
Figure 1.Surfactant molecular aggregates in SC CO2 and ILs. The creation of emulsions or microemulsions with SC CO2 and IL is very interesting. The IL/SC CO2 emulsion [1] and CO2-in-IL microemulsion [2] were successfully formed. And, meso-micro porous metal-organic frameworks were also well prepared as shown in Figure 1. These novel surfactant systems show flexible and controllable characteristics, for example, the properties of CO2 phase can be tuned by controlling the pressure of CO2; the properties of IL phase can be tuned by changing the kind of ILs because of the tunable and designable features of ILs. Therefore, the system composed of the two green solvents is environmentally friendly. SAXS technique was used to reveal the nanoscale structure and confirmed that the size of micelles in microemulsion is indeed tuned by the pressure of CO2. The micellization of amphiphilic molecules is generally tuned by adjusting the temperature and pH value, or adding solid and liquid additives (salt, cosurfactant, etc.). However, the researchers found that the compressed CO2 can induce the micellization of Pluronics at 15 oC [3] . Different from the common micelles with hydrophobic cores, this kind of CO2-induced micelle, interestingly, has an amphiphilic core, and hydrophobic and hydrophilic domain coexistent as shown in Figure 1. SAXS results demonstrate that the micelles are spherical and their size can be tuned by the pressure of CO2. The formation of nanoscale multiple emulsions is very difficult. To induce the formation of nanoscale double emulsion, the ultrasonic and microfluidic homogenization and specially designed surfactants are generally needed.The researchers found that the compressed CO2 can induce the formation of not only water-in-oil-in-water multiple nanoemulsions [4] , but also water-in-oil-in-water double nanoemulsions [5] (see Figure 1). Especially, the formation or devastation of these multiple nanoemulsions can be reversibly controlled by the CO2 pressure. These surfactant molecular aggregates in SC CO2 and ILs have potential applications in material synthesis, chemical reaction and extraction. Published papers: [1] Yueju Zhao, Jianling Zhang, Buxing Han, Jinliang Song, Jianshen Li, Qian Wang, Metal-organic framework nanospheres with well ordered mesopores synthesized in ionic liquid/CO2/surfactant system, Angew. Chem. Int. Ed., 2011, 50, 636-639. [2] Jianling Zhang, Buxing Han, Jianshen Li, Yueju Zhao, Guanying Yang, Carbon dioxide-in-ionic liquid microemulsions, Angew. Chem. Int. Ed.,2011, 50, 9911-9915. [3] Jianling Zhang, Buxing Han, Yueju Zhao, Jianshen Li, Guanying Yang, Switching micellization of Pluronics in water by CO2, Chem. Eur. J., 2011, 4266-4272. [4] Jianling Zhang, Buxing Han, Yueju Zhao, Wei Li, Emulsion inversion induced by CO2, Phys. Chem. Chem. Phys., 2011, 13, 6065-6070. [5] Yueju Zhao, Jianling Zhang, Qian Wang, Jianshen Li, Buxing Han, Water-in-oil-in-water double nanoemulsion induced by CO2, Phys. Chem. Chem. Phys., 2011, 13, 684-689. Beijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Colloid and Interface and Thermodynamics, Institute of Chemistry, Chinese Academy of Sciences (China) *Correspondence: zhangjl@iccas.ac.cn, hanbx@iccas.ac.cn |
|
|
| Chinese
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
News and Events
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility