| Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015 |
| From: PublishDate:2016-06-03 Hits: |
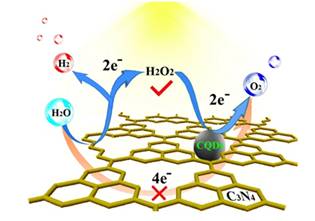
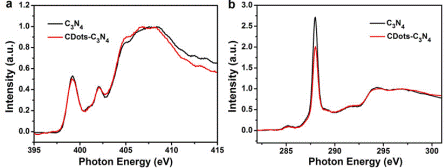
Prof. Kang Zhenhui’ group of Soochow University has published an article “Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway” in Science (Science, 2015, 347(6225), 970-974), and introduced the result on high efficient photocatalyst for stable visible water splitting. This work was selected as the top ten major scientific progresses in China in 2015. Production of hydrogen and oxygen from water using solar energy is a promising technique to develop clean and green energy. In the past forty years, a series of photocatalyst for water splitting were developed. However, it still faces some challenging issues, such as low “solar to hydrogen” (STH) efficiency and poor stability, which impede its practical application. A new type of photocatalyst, a metal-free carbon nanodot–carbon nitride (C3N4) nanocomposite, was reported by Prof. Kang Zhenhui’ group with their collaborators. The catalyst comprises low-cost, Earth-abundant, environmentally friendly materials and shows excellent stability over 200 days. In contrast to the conventional photocatalysts, CDots-C3N4 catalyzes water splitting to hydrogen and oxygen via the stepwise two-electron/two-electron two-step pathway under visible light irradiation.C3N4 is responsible for the first step (photocatalysis) decomposing water into H2O2 and H2, and CDots are responsible for the second step (chemical catalysis) decomposing H2O2 into H2O and O2. X-ray absorption near edge structure (XANES) spectroscopy of C3N4 (black trace) and CDots-C3N4 (red trace) at the N 1s line and at the C 1s line were obtained at 4B7B-Soft X-ray absorption station of BSRF. It demonstrates the interaction between CDots and C3N4 and is useful to explain the photocatalysis mechanism CDots-C3N4. CDots also increase the light absorbance and thus the values of QE and STH. The composite nature of the catalyst provides sufficient proximity between the H2O2 generation sites on the C3N4 surface and the Cdots so that H2O2 decomposition and O2 generation in the second stage become efficient. A recent U.S. Department of Energy (DOE)–solicited technoeconomical analysis of H2 generation by solar water splitting suggested that PC systems with STH = 5% (not far away from the 2% efficiency reported above) would allow a H2 production cost of $2.30/kg, which meets the DOE target of $4/kg H2. The cheapest PE configuration with STH = 10%, in comparison, allows a H2 production cost of $5.60/kg H2, more than twice the cost of the PC system The STH efficiency of CDots-C3N4 photocatalyst is 2% and larger than that previously reported for any stable water-splitting photocatalysts. According to U.S. Department of Energy (DOE)–solicited technoeconomical analysis, catalyst with STH = 2% would allow a H2 production cost of $6/kg which meets the DOE target of $4/kg H2. A further study and development of this new kind of photocatalyst is significant for Production of hydrogen from water using solar energy.
Fig. 1 (up)The mechanism of CDots-C3N4 splitting water using solar energy;(down)X-ray absorption near edge structure (XANES) spectroscopy of C3N4 (black trace) and CDots-C3N4 (red trace) at the N 1s line (a) and at the C 1s line (b). |
|
|
| Chinese
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility