| Characterization of the thermo-reduction process of chalcopyrite at 65 °C by cyclic voltammetry and XANES spectroscopy |
| From: PublishDate:2012-03-15 Hits: |
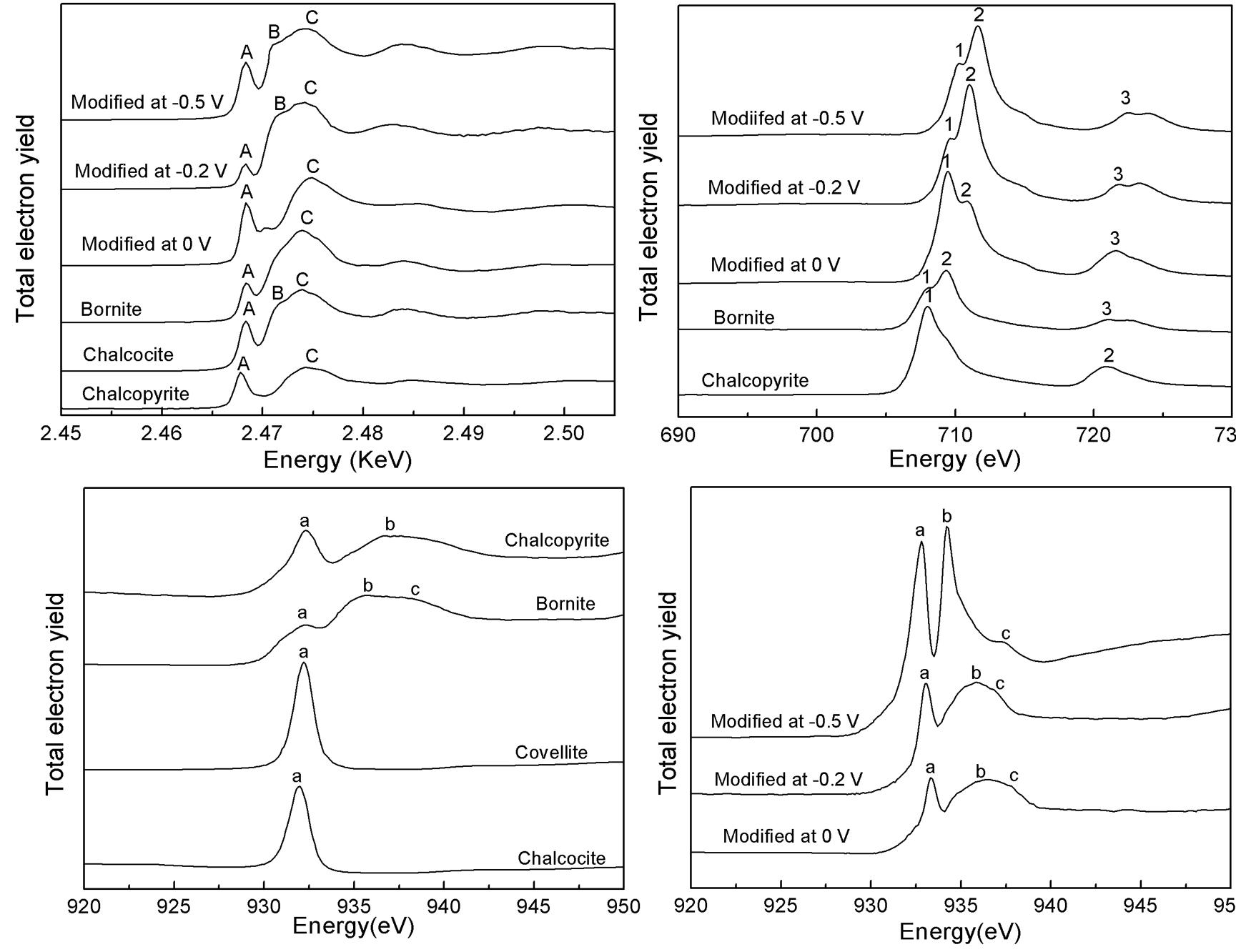
Dissolution of chalcopyrite in most of the hydrometallurgical practices is by using of the oxidative decomposition treatment. However, some researchers reported reducing of chalcopyrite to the more dissolvable copper sulfide chalcocite (Cu2S) may be a feasible alternative treatment. Chalcocite, bornite and metal copper have been proposed as the reduction products of chalcopyrite by electrochemical studies, and the formation of chalcocite has been confirmed in chalcopyrite bioleaching. It is noteworthy that the formation mechanism of these intermediates is difficult to identify by traditional composition analysis method that usually are not able to provide the formation information of these intermediates, and though electrochemical methods can provide the the intermediate reactions information, they cannot provide the direct evidence of the formation of them. A team from School of Minerals Processing and Bioengineering of Central South University, by using of Fe L3-, Cu L3- and sulfur K-edge XANES spectroscopy, had characterized the formation of these reduction products on the chalcopyrite electrode surface which was reduced under special potentials and revealed the transformation conditions of chalcopyrite reduction products.Their research has been published in Hydrometallurgy in April of 2011. The team identified the formation of bornite, chalcocite and metal copper on chalcopyrite electrode surface during chalcopyrite reduction, and the formation of them follow the order of the decrease of the applied potential. The reduction of chalcopyrite to bornite in 0.1 to -0.1 V, and it is the initial and rate-limited step of the successive reduction of chalcopyrite. When the potentials continued moving toward negative direction, the reduction of bornite and chalcopyrite to chalcocite took place in the potential range of -0.1 to -0.56 V. When the applied potential was negative than -0.5 V, the reduction of the remaining chalcopyrite and chalcocite on the electrode surface to elemental copper took place in this negative potential.
Fe, Cu L3-edge and Sulfur K-edge XANES spectra of the massive chalcopyrite electrode potentiostatic reduced at 0, -0.2 and -0.5 V for 240 s, respectively. The results confirmed the formation of bornite and chalcocite during chalcopyrite reduction. The research showed the sulfur K-edge XANES based on synchrotron sources can help to reveal the oxidative and reductive mechanism of sulfur-containing compounds. Instaneous formation of the oxidized and/or reduced compounds can be examined if some in-situ detection facilities are assembled to the synchrotron facilities, which can provide more information and help to reveal the redox mechanisms of the sulfur-containing compounds. Liang Chang-Lia, b, Xia Jin-Lana, b*, Yang Yi a, b, Nie Zhen-Yuan a, b, Zhao Xiao-Juana, b, Zheng Leic, Ma Chen-Yanc, Zhao Yi-Dongc. Characterization of the thermo-reduction process of chalcopyrite at 65°by cyclic voltammetry and XANES spectroscopy. Hydrometallurgy 2011, 107(1-2):13-21. a Key Lab of Biometallurgy of the Ministry of Education of China, Changsha 410083, China b School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China c Beijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China *Correspondence: jlxia@mail.csu.edu.cn |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility