| In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO |
| From: PublishDate:2012-06-26 Hits: |
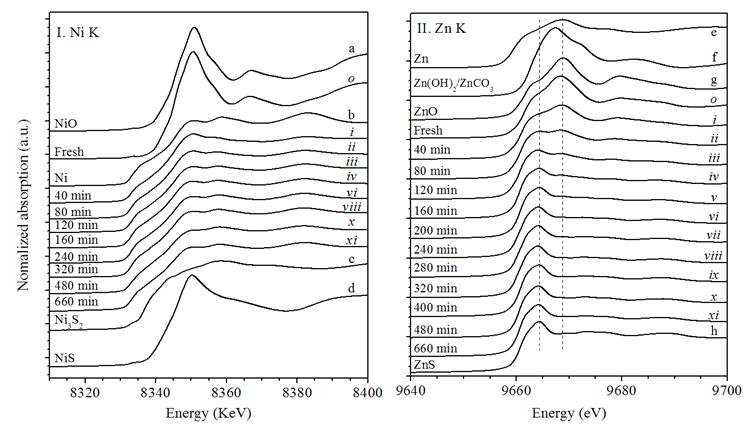
Ni/ZnO is an ideal adsorbent for the reactive adsorption desulfurization (RADS) to produce oil products with ultra-low sulfur content. Though many efforts have been made to reveal the mechanism of RADS, however, current evidences still cannot give a detailed understanding on the transfer mechanism of the sulfur species from oil to the sulfur acceptor, the role of hydrogen in desulfurization, as well as the structure evolution of the adsorbent during the desulfurization process. In this work, the RADS of a model oil n-nonane containing dibenzothiophene (DBT) was conducted over a Ni/ZnO adsorbent. The fresh and spent adsorbents were characterized by X-ray diffraction (XRD), X-ray absorption spectroscopy (XAS), and X-ray photoelectron spectroscopy (XPS); the evolution of Ni/ZnO adsorbent structure during desulfurization was monitored by in situ XAS in Beijing Synchrotron Radiation Facility (BSRF), Institute of High Energy Physics (IHEP), Chinese Academy of Sciences (CAS). Efforts were then made to elucidate the state of active nickel species, the role of hydrogen in desulfurization, the adsorbent structure evolution during the desulfurization process, and the possible reaction mechanism for the RADS process. The results are newly published in Appl. Catal. B: Environmental, 2011, 106(1–2): 26–38. To get a better insight into the evolution of Ni/ZnO adsorbent structure and the transfer of sulfur species from DBT in the feed oil to the adsorbent during the RADS over Ni/ZnO, the desulfurization of the model oil containing DBT was carried out in an in situ XAS cell at 350 °C and 3.0 MPa in the stream of H2–He mixture; meanwhile, a series of XAS spectra were collected in the transmission mode at an interval of 40 min, as shown in Fig. 1. As shown in Fig. 1(I), a series of in situ Ni K–edge XANES spectra are compared with those of fresh calcined NiO/ZnO and the reference samples NiO, Ni, Ni3S2 and NiS. Ni species in the fresh calcined adsorbent is NiO; after carrying out the RADS for 40 min on stream, its white line intensity is greatly decreased and the spectrum becomes quite similar to that of metallic Ni, suggesting that most of NiO is quickly converted to Ni in the reductive reaction atmosphere. During the whole RADS period, metallic Ni is the dominant Ni species and bulk Ni3S2 species is indiscernible even after 660 min on stream, suggesting that a complete sulfidation of Ni is difficult under current reaction atmosphere. Fig. 1(II) presentsthe in situ Zn K–edge XANES spectra of the calcined NiO/ZnO adsorbent during the RADS of the model oil in hydrogen. A change in the chemical state of Zn is also observed during the RADS. Compared with the spectra of the reference samples, it was found that the intensity of the dominant peak attributed to ZnO decreases gradually along with time on stream (TOS), meanwhile the peak attributed to ZnS becomes distinct and its intensity increases incessantly. The gradual evolvement of the XANES spectra with increasing TOS indicates a smooth progression from ZnO to ZnS.
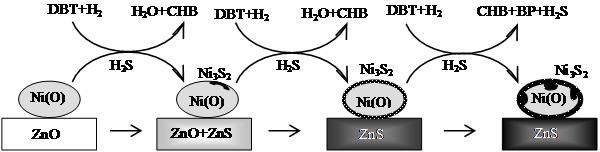
Fig. 1. In situ Ni (I) and Zn (II) K–edge XANES spectra of the calcined NiO/ZnO adsorbent during the RADS of the model oil in hydrogen. The quantitative components of Ni species in the adsorbent, estimated from PCA and LCF of the XANES spectra, indicate that the main Ni-containing components in the adsorbents during the RADS are NiO, Ni and Ni3S2. The content of NiO decreases successively with the TOS, while that of Ni increases at first, and then remains stable until 480 min on stream and thereafter decreases with the TOS. On the other side, the content of Ni3S2 remains almost unchanged before 120 min on stream due to the balance between sulfidation and regeneration; after that, it increases gradually with the TOS for the RADS. Meanwhile, the main Zn components in the adsorbent are ZnO and ZnS; the content of ZnS increases successively, accompanied by a gradually decrease of ZnO content with the TOS. It should be mentioned that the sulfidation rate of ZnO to ZnS decreases with TOS and becomes very low at the later period of the RADS reaction, properly due to the limitation of H2S diffusion into the bulk of unreacted ZnO through a previously formed ZnS layer. Current results suggest that the in situ XAS is an efficient technique in characterizing the structure evolution of a catalysts or adsorbent during the reaction process. Considering also the results of catalytic tests for the RADS, it was found that the desulfurizations under nitrogen and hydrogen are different in the reaction mechanism. In nitrogen, the desulfurization over Ni/ZnO is realized through physical and chemical adsorption; a severe decrease in the desulfurization activity of Ni/ZnO is observed with the TOS and the desulfurization capacity is very low. In hydrogen, the desulfurization turns to be a reactive adsorption process and Ni/ZnO exhibits a high desulfurization activity and capacity. Among the nickel species (NiO, Ni, and Ni3S2) detected in the adsorbent, the metallic nickel is the principal active sites for the RADS over Ni/ZnO. Hydrogen plays an important role in the RADS; it facilitates the decomposition of DBT on the active Ni species, the formation of Ni3S2, and thereafter the transfer of sulfur from Ni3S2 to ZnO. Metallic Ni as the active nickel species is preserved until most of ZnO is converted to ZnS. These observations support the three-step sulfur transfer mechanism for the RADS over Ni/ZnO in hydrogen (Fig. 2): DBT in the model oil is first decomposed on surface Ni of Ni/ZnO to form Ni3S2, then Ni3S2 is reduced in the presence of hydrogen to form H2S and the active nickel species is liberated from Ni3S2, and lastly H2S is stored in the adsorbent accompanied by the conversion of ZnO into ZnS. It seems that the presence of hydrogen is favorable and even essential for all three steps. Because the nickel species and zinc species are well contacted in the coprecipitated adsorbent and the in situ intermediate H2S species are highly active, the sulfur transfer is efficient during the RADS process in the presence hydrogen.
Fig. 2. Proposed mechanism for the RADS of DBT-containing model oil over the Ni/ZnO adsorbent in hydrogen.
Article: Lichun Huang, Guofu Wang, Zhangfeng Qin*, Mei Dong, Mingxian Du, Hui Ge, Xuekuan Li, Yidong Zhao, Jing Zhang, Tiandou Hu, Jianguo Wang*,In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO,Appl. Catal. B: Environmental, 2011, 106(1–2): 26–38. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility