| A 5-coordinated adsorbed Zn found at water-TiO2 interfaces |
| From: PublishDate:2012-06-26 Hits: |
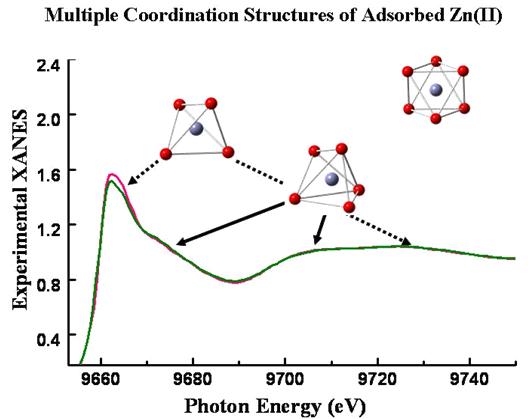
The local structure of aqueous metal ions on solid surfaces is central to understanding many chemical and biological processes in soil and aquatic environments. Differences in surface coordination structure may greatly affect the local chemical properties, long-range interactions, surface reactivity, and bioavailability of metal ions in the aquatic environment. Conclusive diagnosis of the surface coordination structures of metal ions on the atomic scale is helpful for the understanding of the toxicity and environmental impacts of the pollutants. A team from Research Center for Eco-Environmental Sciences (RCEES), CAS, has gained insight into the coordination structures of hydrated Zn(II) on TiO2 surfaces obtained by XAFS spectroscopy. Their research has been published in January, 2011 in Environmental Science & Technology. The team identified the local coordination structure of hydrated Zn(II) at water-TiO2 interfaces using EXAFS and XANES spectroscopy combined with DFT calculations. A 5-coordinated geometry of adsorbed Zn(II) on TiO2 surfaces was reported for the first time. They found that the multiple coordination structure was due to the coexistence of 4-coordinated and 5-coordinated adsorption states. The spectral and theoretical diagnosis of coordination properties would terminate the pending controversy and hypothesis about the coordination chemistry of Zn(II) cation, and may lead to re-examination on pollutants from atomic scale. Zn(II) may occur in 4, 5, and 6-oxygen coordinated sites in different adsorption states, and the coexistence of different adsorption states formed the multiple coordination environments. The XAFS analyses were performed on the beam lines 4W1B of the Beijing Synchrotron Radiation Facility (BSRF). The research provided a new interpretation of the EXAFS data using DFT calculations and experimental XANES analysis for metal ion adsorption on oxide surfaces. The coordination complexity of adsorbed Zn(II) suggested a potential new development of surface complexation models that can take into account the microscopic coordination structures in describing macroscopic relationship between equilibrium concentrations in solution and on solid surfaces. The atomic-level identification of surface coordination geometry helps improve our understanding of the toxicity and environmental impacts of metal ions, because the change of metal coordinationstructure may produce a significant impact on its speciation and transport in the environment.
Article: Guangzhi He, Gang Pan*, Meiyi Zhang and Glenn A. Waychunas,Coordination Structure of Adsorbed Zn(II) at Water-TiO2 Interfaces, Environ. Sci. Technol.,2011, 45(5), 1873–1879. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility