| Dissolution and Microstructural Transformation ofZnO Nanoparticles under the Influence of Phosphate |
| From: PublishDate:2013-06-15 Hits: |
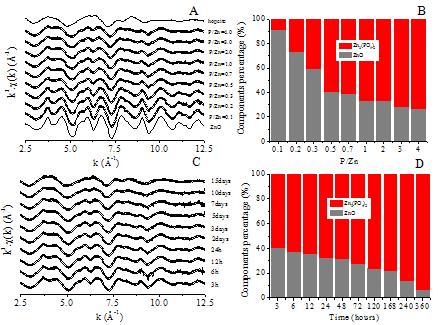
Because of the large quantities produced and their widespread application, manufactured nanoparticles (NPs) will inevitably enter the environment, and it is estimated that their release to the environment will increase drastically in the near future. Toxicity and fate of NPs have drawn great research attention. Nevertheless, lack of the knowledge on their behaviors, particularly their speciation transformation, will inevitably limit our understanding on their toxicity and fate. Due to their high reactivity, it is unlikely that engineered NPs will remain in their original form after release into the environment, and environmental components will inevitably interact with NPs and influence their physicochemical properties and microstructure and consequently determine their potential toxicity and fate in the environment. It is a fact that NPs are difficult to be separated from the environment mediums, therefore in situ spectral analysis of speciation and microstructure of NPs in the environment is highly demanded, and synchrotron based X-ray absorption spectroscopy is well suited for such purpose. A team from Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, used synchrotron based X-ray absorption fine structure spectroscopy (XAFS) combining with some other technologies such as ICP-MS, X-ray diffraction (XRD) and high resolution transmission electronic microscopy (HRTEM) to analyze the speciation and microstructural transformation of ZnO NPs in the presence of phosphate which exist widely in aqueous environment. Their research has been published on May 31st, 2012 in Environmental Science & Technology. The results show that phosphate at a low concentration rapidly and substantially reduce the release of Zn2+ into aqueous solution. Toxicity of ZnO NPs is considered mainly due to dissolved Zn ions and therefore would be greatly reduced in the presence of phosphate. TEM observation shows that uniform spherical nanoparticles almost disappeared and anomalous porous particles of much larger size formed in the presence of phosphate. HRTEM, XAFS and XRD analyses reveal that speciation of Zn transformed from ZnO to zinc phosphate including hopeite and parahopeite. In addition, mixed amorphous and crystalline phases of ZnO and zinc phosphate are generated in this process. Such phosphate-induced transformation will significantly influence the behavior and fate of ZnO NPs in the environment.
X-ray absorption spectra of ZnO NPs treated at difference phosphate concentrations and time were collected at the 1W1B beamline of the Beijing Synchrotron Radiation Facility, which indicated that ZnO NPs transformed from ZnO to Zn3(PO4)2.4H2O in the presence of phosphate. To our knowledge, this is the first study in which the detailed process of phosphate-induced speciation and microstructural transformation of ZnO NPs has been analyzed. In view of the wide existence of phosphate contamination in water and its strong metal-complexation capability, phosphate-induced transformations may play an important role in the behaviors, fate and toxicity of many other metal based nanomaterials in the environment. Furthermore, phosphate is an essential component of many culture media used in the nanotoxicity testing. Therefore it is important to take into account the presence and concentration of phosphate in the surrounding environment when evaluating the toxicity of NPs.
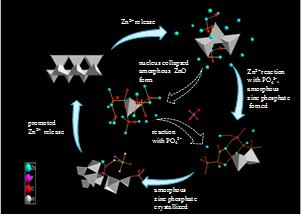
Summary of the transformation process of ZnO NPs
Article: Jitao Lv, Shuzhen Zhang*, Lei Luo, Wei Han,Jing Zhang, Ke Yang, Peter Christie, Dissolution and microstructural transformation of ZnO nanoparticles under the influence of phosphate. Environmental Science & Technology, 46 (3): 7215-7221, 2012. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility