| Nanopillars by CalciumChloride Self-assembly and Dry Etching |
| From: PublishDate:2013-06-15 Hits: |
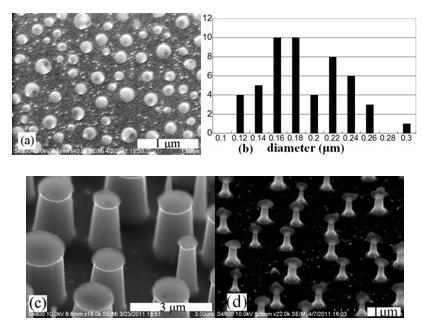
With the development of nanotechnology, nanopillars have been sought after for many applications, such as sensors, solar cell’s antireflection, Li ion battery negative electrodes, etc. Fabrication methods for nanopillars, based on dry etching with self-assembly masks, may be classified as four types: metal, nanospheres, copolymer, and deliquescent salts. Evaporation coated thin film of deliquescent salts may aggregate into nanodots in wet air, with advantages of easy mask removing, good coverage over uneven surface, wide diameter range and compatibility. So far, Cesium Chloride (CsCl) was the only one successful salt, based on CsCl diffusion in absorbed water layer. Herein, the researchers from X-ray lithography station of BSRF proposed a new material CalciumChloride (CaCl2), driven by exothermal reactions with absorbed waterto nano dots and then fabricated silicon or PolymethylMethacrylate (PMMA) nanopillars by Inductively Coupled Plasma (ICP) dry etching with CaCl2 dots as mask. Their research has been published on September 25th, 2012 in Materials Letters. CaCl2 film is coated on silicon wafer or PMMA by thermal evaporation in vacuum around 0.01Pa; then it is exposed to air of certain relative humidity around 20℃. Immediately (within one second), the film aggregates into discontinuous dots. Next, ICP dry etching transfers the dots into silicon or PMMA pillars. Finally, residual CaCl2 is removed by water soaking for 1min. This technique is easy, tunable andcost-effective, which is possibly controlled within 20min. CaCl2 self-assembly is based on the exothermal reaction of CaCl2 and H2O, from which the released heat gathers CaCl2 together. CaCl2 firstly combines with two water molecules to form dihydrate, and then gradually absorbs more to become tetrahydrate or hexahydrate, leading to the change of CaCl2 dots’ topographies along with time. The average diameter, coverage ratio (area covered by dots to Si surface) and topography of CaCl2 dots are controlled by film thickness, substrate moisture content, and air relative humidity. For certain thickness, more heat, either from gas water or substrate moisture, can move more CaCl2 molecules to pack more centrally, forming larger dots with smaller coverage ratio. For certain heat, the thicker film is, the smaller fraction of moved mass is, leading to larger diameter, greater coverage ratio, and flatter topography.The self-assembly nano dots are disordered and near Gaussian in size distribution and the coverage ratio on Si wafer can achieve to 37%.Moreover, CaCl2nano dots can keep integrity for more than one month in arbitrary relative humidity at room temperature, stable enough for practical use. With these nano dots as masks, different average diameters and heights nanopillars can be fabricated by different ICP dry etching. Evaporation coated CaCl2 can cover uneven surface and make the nanopillars planted at unpolished Si wafer to avoid high polishing cost. The figures show CaCl2 nano dots, Si and PMMA nanopillar arrays.
Figures: (a) CaCl2 dots with about 200nm average diameters on PMMA; (b) the diameters distribution of (a); (c) Si nanopillars with 1.4μm average diameter and 2μm height; (d) PMMA nanopillars of 200nm average diameter and 1μm height. This research provides us with a new method of fabricating nano masks and nanopillars. Compared with conventional CsCl materials, the CaCl2self-assembly technology is more stable, easier, faster which could add a new option in self-assembly nanomasks.
Article: Yuanxun Liao, Jing Liu, Bo Wang,and Futing Yi*, Nanopillars by calcium chloride self-assembly and dry etching.Materials Letters, 67, 323–326, 2012. |
|
|
| Chinese
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility