| From {Au(I)...Au(I)}-coupled cages to the cage-built 2-D {Au(I)...Au(I)} arrays: Au(I)...Au(I) bonding interaction driven self-assembly and their Ag(I) sensing and photo-switchable behavior |
| From: PublishDate:2015-06-16 Hits: |
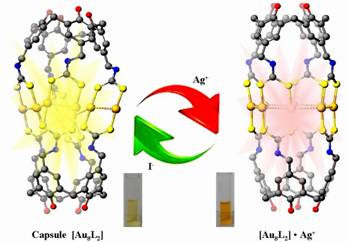
Since the recognition of the multiple bond in [Re2Cl8]2- by F. A. Cotton in 1960s, the chemistry of the dimetal- or multimetal-centered compounds has drawn increasing interest due to their structural diversity, rich physical properties and potential applications in catalysis, biological mimicry, magnetic coupling, electron transfer, functional materials, etc. Using a AuI···AuI bonding interaction-directed stepwise macrocyclization strategy, Yu and co-worker has developed a series of giant ring systems from Au12 to Au16 and Au36 with novel luminescence properties in some cases(related work have been published in the important journals including J. Am. Chem. Soc.2005, 127, 17994-17995. Also see: Editors’ Choice by P. D. Szuromi, Science 2005, 310, 1745 Angew. Chem. 2008, 120(24), 4627-4630; Angew. Chem. Int.Ed. 2008, 47(24), 4551–4554 (VIP and Cover Picture). Based on previous work, we herein report a new class of gold(I)-containing metallo-organic cages {Au8L2} (L = tetrakis-dithiocarbamato-calix[4]arene, TDCC), 1–3, which are self-assembled from two deep-cavitand calix[4]arene-based dithiocarbamate ligands and eight Au(I) metal centers featuring a quadruple-stranded helicate structure. Interestingly, these functional supramolecular metallo-cages aggregate into two-dimensional sheet-like molecular arrays in the solid state, consisting of one-dimensional molecular wires with extended intra- and intermolecular AuI···AuI bonding interactions. The electronic absorption and emission spectra of complexes 1–3 confirm that the programmable self-assembly process occurs in a stepwise manner with the links built via aurophilic interactions. Photophysical investigations in the solution state revealed that the gold(I) molecular capsules could function as highly selective and sensitive phosphorescent sensors for silver ions and reversible light-convertible ion switches. These cages maybe used as a proof of concept metallo-supramolecular chemosensors and published in the Journal of the American Chemical Society, 2014, 136 (31), 10921–10929.
We design and synthsized a novel kind of tetra-dicarbodithiolatecalix[4]arene ligands L1-L3 with different conformation via multi-step synthesis. By employing these function ligands reaction with linear assembling unit Au(THT) in solution, finally leading to the formation of a new class of two-dimensional gold (I)-containing molecular wires supramoleculars 1 and metallosupramolecular cage 2 {Au8L2}n (n=1 or ∞; L=tetra-dicarbodithiolatecalix[4]arene, TDTC). These novel supramolecular capsules were depth self-assembled through inter- and intramoleculer AuI…AuI bonding based on 3-D flexible calix[4]arene-based supramolecular cages [Au8L2] consisting of octa-memberd Au(I) metal centers. Synchrotron radiation analysis of the complex 1-3 revealed the presence of cages with a pseudo C4v symmetry. For these complexes, the crystallography indicated that the structure of gold (I) supramolecular cages as the building blocks was the quadruple-stranded helicate dimer. The 2-D AuI…AuI bonding molecular wire networks consisting of two types of chains [Au8]n (n=1, octa-atomic chains Au8 and n=∞, infinite chains [Au8]n) were built from two neighboring supramolecular cages Au8L2 via aurophilic bonding and linked by bridging polydentate calix[4]arenes ligands. It's almost the same size with cage 1, the length from end to end is about 17.86Å (O11-O15), average distance between two four-membered AuI…AuI chains is 6.84 Å which become a slightly shorter than cage 1.The 2-D molecular wires containing aurophilic attraction exhibited green luminescence at 624 nm in the solid state and 550 nm in the CH2Cl2 at 298 K, respectively, which were assigned to the 3MC and 3LMCT or 3LMMCT between the Au(I) centers and organic cavitand ligands. Two types of one- dimensional continues metal-metal bonding chains Au8 and [Au8]n with potential internal cavity possibly used as molecular container for appropriate guest molecules encapsulated within the spheroidal carceplex. Electronic absorption and emission studies of complexes 1–3 indicated the occurrence of a programmable self-assembly process in a concentration-dependent stepwise manner with the links built via aurophilic interactions. These novel gold(I) supramolecular cages exhibited green phosphorescence and have been shown to serve as highly selective and sensitive luminescent sensors toward AgI cation among various competitive transition metal ions. Such supramolecular assemblies exhibit potential applications in host-guest chemistry, artificial enzyme, molecular recognition, and may serve as selective luminescent materials for sensors and OLEDs. This work was highly evaluated by international peers with the praise word of “highly origin concept”. At this time, we warmly thank professor Yam of HKU for their great work on the opt-electronic function determination and important discussion. |
|
|
| Chinese
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility