| Atomic cobalt on nitrogen-doped graphene for hydrogen generation |
| From: PublishDate:2016-06-03 Hits: |
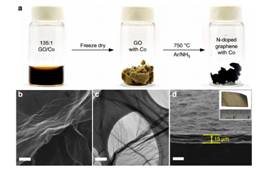
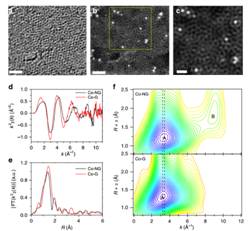
The hydrogen economy represents a future economic plan and has raised lots of concerns. The ability to generate molecular hydrogen (H2) in a clean, sustainable and cheap way is essential to the success of the proposed hydrogen economy. One established method for hydrogen generation is electrochemical water splitting, in which water is converted into molecular hydrogen by means of an electric current; however, the large reaction kinetic barrier (or overpotentials) makes its commercialization for mass production depending critically on the development alternative highly active while inexpensive catalysts to replace the noble metal platinum. The researchers from Rice University, the Beijing Synchrotron Radiation Facility, the University of Texas at San Antonio and the University of Houston developed a novel hydrogen generation catalyst based on trace of cobalt and graphene, which has proven nearly as effective as platinum-based catalysts. The research results have been published in September, 2015 in Nature Communications. The researchers found that small amounts of cobalt atoms (~0.5 at%), coordinated to nitrogen atoms on the grapheme, is highly active toward hydrogen generation with 30 mV onset overpotential and it takes only ~150 mV to deliver current density of 10 mA cm-2. In addition, this catalyst is stable in both acidic and basic electrolytes. Instead of nanoparticles or nanoclusters, these cobalts were found to be single atoms uniformly dispersed on graphene nanosheets, as revealed by aberration-corrected STEM, indicating that the special existing forms of Co atoms is crucial to achieve such high activity. Further EXAFS analyses obtained by qualitative wavelet-transform (WT) and quantitative Fourier-transform reveal that the atomic cobalt is bonded to the neighboring nitrogen atoms, which suggests that the catalytically active sites are associated with the metal centers coordinated to nitrogen. Besides, the large structural disorder implies that these active sites are associated with many structural defects and disorder. The exact structure and roles of the defect remain unclear and its determination would need more thorough studies of structural characterization techniques. Figure. 1 (Left) Schematic illustration of the synthetic procedure of the Co-NG catalyst and morphology characterizations by SEM and TEM. (Right) Bright-field aberration-corrected STEM image of the Co-NG and wavelet-transform analysis of the EXAFS results for Co-NG and Co-G. The nitrogen-doped graphene, incorporated with very small amounts of Co as individual atoms, represents the first example of single-atom catalysis (SAC) achieved in inorganic solid-state catalysts for the hydrogen evolution reaction (HER), and the excellent catalytic performance, maximal efficiency of atomic utility, scalability and low-cost for the preparation makes this catalyst a promising candidate to replace Pt for water splitting applications. In addition, the approach demonstrated in this work in obtaining individual metal atoms that are supported on graphene and the use of XAFS technique to explore the active sites may be a harbinger for broad applicability of this methodology for other atomic-scale catalytic systems. The research results have been reported by many website such as Nature Energy, Phys.org, R&D, and Nanotechnology Now. Article: Huilong Fei, Juncai Dong, M. Josefina Arellano-Jiménez, Gonglan Ye, Nam Dong Kim, Errol L.G. Samuel, Zhiwei Peng, Zhuan Zhu, Fan Qin, Jiming Bao, Miguel Jose Yacaman, Pulickel M. Ajayan, Dongliang Chen2 & James M. Tour, Atomic cobalt on nitrogen-doped graphene for hydrogen generation, Nat Commun 6, 8668 (2015). |
|
|
| Chinese
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility