| In situ detection of calcium phosphate clusters in solution and wet amorphous phase by synchrotron X-ray absorption near-edge spectroscopy at calcium K-edge |
| From: PublishDate:2016-06-03 Hits: |
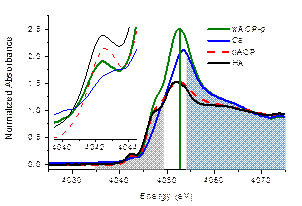
Amorphous calcium phosphate (ACP) is a precursor phase frequently present at the early stage of crystallization from aqueous solution. According to a structure model proposed by American scientists in 1970s, the amorphous phase is the aggregates of clusters with the composition of Ca9(PO4)6 and water molecules filling in the interstitial space. Recently, a team from Peking University School of Pharmaceutical Sciences have made progress in the study of the structure of amorphous calcium phosphate. Their results have been published in Crystal Growth & Design on May 17, 2015. On the Beamline 4B7A at Beijing Synchrotron Radiation Facility (BSRF), the team prepared amorphous calcium phosphate on-site and examined the wet sample using the X-ray absorption near-edge spectroscopy at calcium K-edge. As shown in the following diagram, the wet amorphous phase (wACP) exhibits a dual character in its short-range order: Some of its clusters are similar to hydrated calcium ions (Ca), and some others to those in crystalline hydroxyapatite (HA). These spectral features are quite different from those of dry amorphous phase (dACP) that shows close resemblance to hydroxyapatite in the short-range order throughout the whole spectrum, well described by the early structure model.
The dual character of the wet amorphous calcium phosphate in Ca K-edge XANES spectrum. Ca: Hydrated Ca2+ ions at 4.0 mmol/L; wACP-p: The precipitate of wet amorphous calcium phosphate; HA: crystalline hydroxyapatite; dACP: dry amorphous calcium phosphate. This finding provides a basis for a better understanding and rational control of calcium phosphate crystallization at the molecular level. Upon formation in the aqueous solution, calcium phosphate clusters aggregate and cross-link through the bridging phosphates. As the “molecules” become larger and denser, an amorphous phase finally emerges. The Ca-to-P ratio of the latter is strongly dependent on the initial composition of the solution. By releasing hydrated phosphates, the amorphous phase attains a higher Ca-to-P ratio and a lower water content, changing toward a thermodynamically stable crystalline phase. Professor Tian-Lan Zhang, the team leader, comments on their progress: “The formation of calcium phosphate solid in human body, such as bone metabolism and vascular calcification, is mediated by proteins. Affected by the amino acid residues on the backbone chain, the carboxyl and phosphate groups in a protein molecule are less hydrated than the corresponding free anions in solution. Consequently, they can bind Ca2+ ions and form clusters containing less water molecules, acting as seeds and incurring subsequent structural changes toward crystallization. In this sense, our basic study is associated to biomedical science. ” Article: Qun Zhang,* Yun Jiang,* Bao-Di Gou, Jian Huang, Yu-Xi Gao, Jia-Ting Zhao, Lei Zheng, Yi-Dong Zhao, Tian-Lan Zhang,** and Kui Wang. In situ detection of calcium phosphate clusters in solution and wet amorphous phase by synchrotron X-ray absorption near-edge spectroscopy at calcium K-edge. Cryst. Growth Des. 2015, 15, 2204-2210. |
|
|
| Chinese
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility