| New insight into coordination and extraction of Uranium(VI) with N-donating ligands in Room Temperature Ionic Liquids |
| From: PublishDate:2016-06-03 Hits: |
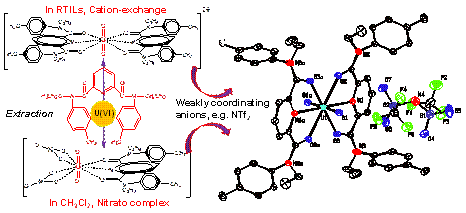
Room temperature ionic liquids (RTILs) represent a new class of solvents with ionic and liquid character at or around room temperature, showing ever-increasing potential applications in radiochemical separation, in particular, in liquid/liquid extraction of nuclear fuel associated radionuclides from aqueous solutions. The related coordination chemistry and related extraction mechanisms, however, are still not well understood and remain deep fundamental interest. Laboratory of Nuclear Energy Chemistry from Multi-discipline Research Center in Institute of High Energy Physics recently conducted a study in which the detailed structure of an extracted U(VI) species in RTILs was firstly identified based on synchrotron radiation analysis, and the the inherent relationship between weakly coordinating anions from RTILs and U(VI) coordination/ extraction with N-donating ligands were indicated. Their research has been published on February 28th, 2015 in Inorganic Chemistry. The team performed the solvent extractions of uranium(VI) from nitric acid media by two commonly used RTILs, i.e. 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]) and 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)-imide ([C4mim][[NTf2]), in combination with N,N’-diethyl-N,N’-ditolyldi- picolinamide (EtpTDPA) as extractant. The extraction mechanism and the complexation of uranium(VI) with EtpTDPA in the RTILs were investigated in detail. The results suggest that the EtpTDPA/RTILs system is an effective media for of uranium(VI) extraction with high efficiency ( > 95%) and rapid kinetics (< 4 min), and the extraction most likely occurs by a cation-exchange mode since the concentration of C4mim+ in the aqueous phase increases linearly with the uranium(VI) extraction evidenced by UV-VIS measurement. More interestingly, the uranium(VI)/EtpTDPA complex in RTILs precipitated immediately after the extraction. After recrystallization from acetone, the precipitates were characterized by synchrotron radiation-based XRD, revealing a composition of [UO2(EtpTDPA)2[NTf2]2 or [UO2(EtpTDPA)2[PF6]2 (Fig.1). FTIR and EXAFS analysis confirmed that the extracted U(VI) species in RTILs have the same structure with the precipitates. This is the first case of crystallographically identification of the extracted U(VI) species in RTILs. Moreover, it is found that the extracted species in RTILs are greatly different from that in dichloromethane (identified as UO2(NO3)2(EtpTDPA)]), suggesting the particularity of RTILs-based extraction systems. Further studies show that the complex formation in RTILs is independent of the cation-exchange since incorporating UO2(NO3)2, EtpTDPA and LiNTf2 or KPF6 in a solution also produces the same complex as that in RTILs, revealing the important roles of weakly coordinating anions on the coordination chemistry between U(VI) and EtpTDPA.
Fig.1 The dependence of the coordination chemistry between U(VI) and EtpTDPA on weakly coordinating anions This work suggest that cation-exchange extraction mode for ILs-based extraction system probably originates from the supply of weakly coordinating anions from RTILs, and the coordination of uranium(VI) with EtpTDPA and/or other extractants, as well as the cation-exchange extraction mode could be potentially changed by varying the counter ions of uranyl or introducing extra anions. In this work, synchrotron radiation technique play a key role by helping the team to identify the extracted U(VI) species. Article: Li-Yong Yuan, Man Sun, Lei Mei, Lin Wang, Li-Rong Zheng, Zeng-Qiang Gao, Jing Zhang, Yu-Liang Zhao, Zhi-Fang Chai* and Wei-Qun Shi* New insight of coordination and extraction of Uranium(VI) with N-donating ligands in Room Temperature Ionic Liquids: N,N’-diethyl-N,N’-ditolyldipicolinamide as a case study. Inorganic Chemistry 54 (2015), 1992−1999. |
|
|
| Chinese
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility