| Exploring the coordination change of vanadium and structure transformation of metavanadate MgV2O6 under high pressure |
| From: PublishDate:2017-06-16 Hits: |
Vanadium oxide-based compounds have received considerable attention owing to their outstanding physical and chemical characters which originated from their diversified crystal structures, the various valance states of the vanadium in the compound, and the different V-O coordination spheres. Consequently, they find extensive industrial applications including phosphors, gas sensors, catalysis, rechargeable batteries, and also in electrochemical devices. A prominent example is that MgV2O6 with brannerite structure shows a high charge capacity when used as anode material for lithium ion rechargeable batteries. The synthesis method and phy-chemical properties have been well investigated at ambient condition. However, the structural stability and the coordination change of vanadium in MgV2O6 under high pressure have not been studied. Two teams from State Key Laboratory of Superhard Materials of Jilin University and Center for High Pressure Science and Technology Advanced Research have gained insight into the structure transformation, the coordination change of vanadium, and electrical transport property under high pressure. Their research has been published on December 7th, 2016 in Scientific Reports.
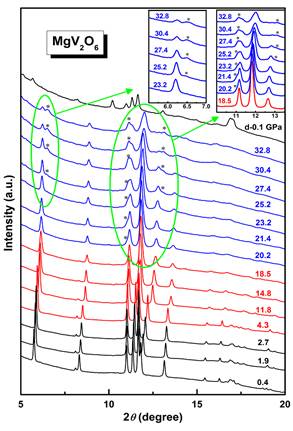
Figure 1. Selected high pressure ADXRD patterns of MgV2O6. Insert shows the appearance of the new peaks. Raman spectroscopy, synchrotron angle-dispersive X-ray diffraction (ADXRD), first-principles calculations, and electrical resistivity measurements were carried out under high pressure to clarify the structural stability and electrical transport properties of MgV2O6. The powerful synchrotron radiation at BSRF shows that the coordination number of vanadium ions changes from 5+1 to 6 and the distorted octahedral changes to a more rigid one around 4 GPa. This phenomenon is different from the increase in coordination number of vanadium, which is caused by the conversion from tetrahedral VO4 to octahedral VO6 as occurred in InVO4 under pressure. Generally the different catalytic properties of bulk and supported vanadia catalysts can usually be related to modifications in the coordination and environment of the V species, which can modify its redox properties. Therefore, the changes of V ions’ coordination number in MgV2O6 may lead to modify its redox properties. With further compression, a structural phase transition from C2/m to C2 phase occurred around 20 GPa. This phase transition process arises from the enhanced distortions of MgO6 octahedra and VO6 octahedra under high pressure. Furthermore, electrical resistivity decreased with pressure but exhibited different slope for different phases, indicating that the pressure-induced structural phase transitions of MgV2O6 results in obvious changes in its electrical transport behavior.  Figure 2. Structure of the C2/m phase at (a) 1.9 GPa, (b) 4.3 GPa, and the C/2 phase at (c) 27.4 GPa, obtained from the Rietveld refinements of the XRD patterns. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility