| Chromium uptake and translocation in arbuscular mycorrhizal symbiosis |
| From: PublishDate:2017-06-16 Hits: |
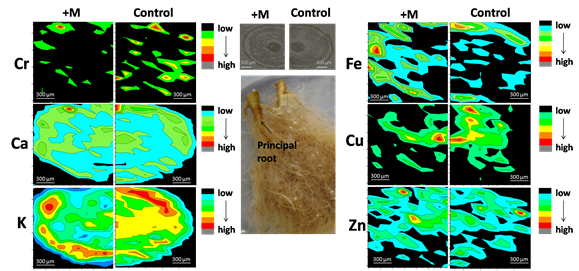
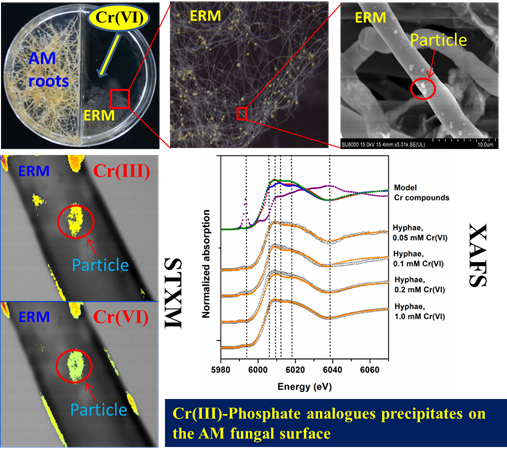
Arbuscular mycorrhizal fungi (AMF) are ubiquitous soil fungi that form symbiotic relationships with more than 80% terrestrial plants. The fungi provide mineral nutrients (especially phosphorus) and water to their plant partner, and in return they obtain carbohydrates from the host plants. It has been demonstrated that AM symbiosis can significantly enhance plant survival under Cr contamination, and thus has a great potential in phytoremedation of soils contaminated with Cr. However, the detailed processes of Cr uptake and translocation in plant-AMF symbioses are still unclear. Recently, a research group from Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences has investigated the chromium uptake and translocation in the plant-AM fungi continuum. The results have been published in Environmental and Experimental Botany (2016, 122: 10–18) and Journal of Hazardous Materials (2016, 316: 34–42). In their previous experiments, AM fungal inoculation could increase plant P uptake, and thus relieve Cr phytotoxicity. Further study found that only P application could not increase plant dry weights as well as AM inoculation did. By applying X-ray fluorescence micro-spectroscopy (μ-XRF, Beijing Synchrotron Radiation Facility, beamline 4W1B) analysis, they found that Cr was mainly located in vascular bundle and cortex in the principal roots without AMF inoculation, while Cr was only located in cortex in principal roots when the plant was mycorrhizal (Figure 1). This may indicate that, besides enhancement of P acquisition, AM symbiosis can also stabilize Cr in mycorrhizal roots and thus restrict Cr transport to shoots. Furthermore, by using a three-compartment cultivation system, they demonstrated that extraradical mycelium (ERM) of AM fungi could take up and transport Cr to mycorrhizal roots from distance, but might not translocate these Cr from roots to shoots, and therefore contributed to Cr stabilization in mycorrhizal roots (Wu et al., 2016. Environmental and Experimental Botany 22:10-18). In order to uncover mechanisms of Cr stabilization by AM fungi, a two-compartment root-organ cultivation system was employed to study the direct interaction between AM fungi and chromium. Through various microspectroscopic analysis e.g. FE-SEM-EDS, scanning transmission soft X-ray microscopy (STXM, Shanghai Synchrotron Radiation Facility, beamline BL08U1A) and X-ray absorption fine structure (XAFS, Beijing Synchrotron Radiation Facility, beamline 1W1B) techniques, they confirmed that AM fungi could reduce Cr(VI) to Cr(III), and precipated Cr(III) with phosphate groups on the fungal surface (Figure 2). The immobilization of Cr by extraradical mycelium of AM symbioses restrained the translocation of Cr into plant root cells, and thus relieved Cr phytotoxicity (Wu et al., 2016. Journal of Hazardous Materials 316:34-42). These studies have uncovered the processes of Cr translocation and transformation in AM symbioses, which can not only contribute to understanding the mechanisms of the positive role of AM symbiosis in plant Cr tolerance, but also provide new insights into biogeochemistry of Cr in AM fungal-plant system. Besides, the studies also provide support for development of mycorrhizal based bioremediation techniques.
Figure 1. Distribution of chromium (Cr) and mineral nutrients (Ca, K, Fe, Cu and Zn) in the thin sections of the principal roots of dandelion plants (Taraxacum platypecidum) inoculated with/without AM fungus under 10 mg kg-1 Cr(Ⅵ) contamination as revealed by SR μ-XRF mapping at the microscopic scale. Pixel size is 50 μm.
Figure 2. Processes and mechanisms of Cr(VI) reduction and precipitation on arbuscular mycorrhizal (AM) fungal surface. Article: Songlin Wu, Xin Zhang, Baodong Chen*, Zhaoxiang Wu, Tao Li, Yajun Hu, Yuqing Sun, Youshan Wang. Chromium immobilization by extraradical mycelium of arbuscular mycorrhiza contributes to plant chromium tolerance. Environmental and Experimental Botany 122 (2016): 10–18. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility