| Dual Electrical-Behavior Regulation on Electrocatalysts for Enhanced Hydrogen Evolution Reaction | ||||||
| From: PublishDate:2017-06-16 Hits: | ||||||
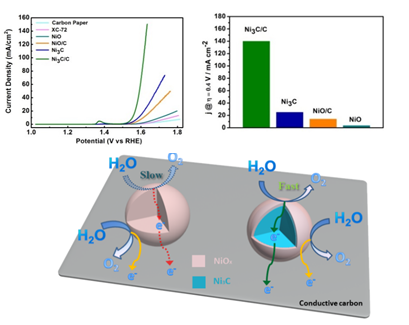
The electrochemical splitting of water is one of the most efficient pathways for H2 production. Developing oxygen evolution reaction (OER) electrocatalysts is the fundamental issue for realizing this promising method of sustainable energy storage. Recently, to improve the catalytic efficiency of cost effective OER electrocatalysts, researchers around the world have been engaged in maximizing exposure active sites of these materials by designing specific morphology. However, few studies focus on improving the oxygen evolution performance by modulating the electronic transport behavior of electrocatalysts. Previous studies have found that the catalytic activity of materials can be significantly optimized by hybridizing semiconductor electrocatalyst material with conductive substrate, such as carbon black, carbon nanotubes, graphene, etc. Performance enhancement of these hybrid catalysts arises from facile interface charge transfer around electrocatalyst. Recent advances have seen that metallic OER electrocatalysts show superior catalytic activity than the semiconducting/insulating catalysts, owing to faster electron transport. However, it remains open questions how to synergistically regulate the electron transport both inside and on the surface of the electrocatalyst and whether dual electrical behavior regulation on electrocatalysts can achieve a higher catalytic activity. Professor Changzheng Wu and co-workers from University of Science and Technology of China, reported that dual electrical-behavior regulation based on metallic electrocatalyst can successfully improve its corresponding catalytic activity. Related results published on Advanced Materials (Adv. Mater. 2016, 28 (17), 3326–3332).
They reported a new electrocatalyst, i.e. Ni3C/C, a hybrid structure of Ni3C metallic nanoparticles and conductive carbon, and found that the intrinsically metallic character of Ni3C was beneficial to electron transport inside electrocatalysts, along with conductive carbon also facilitated the charge transfer on catalyst surface. Benefited from synergistic regulation of electrical transport, Ni3C/C performs the best OER activity among the serial catalysts, including Ni3C, NiO/C and NiO. For example, at the overpotential of 0.4 V, the OER current density of Ni3C / C is up to 140 mA / cm2, which is 5.6 times, 10 times and 40 times of Ni3C, NiO/C and insulating NiO.
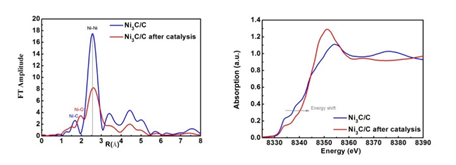
X-ray absorption fine structure spectroscopy (XAFS) was performed before and after the electrochemical tests at the Beijing Synchrotron Radiation Facility (BSRF) 1W1B line station. As shown in the absorption X-ray absorption near-edge structure (XANES), the Ni K spectrum line of Ni3C move to the high energy distinctly after the catalysis, indicating the increase of valence state in Ni. In addition, the results of extended x-ray absorption fine structure (EXAFS) suggested that the Ni-O bonds emerged after the catalysis, and the Ni-Ni bond strength was decreased and the bond length was increased. They also provided in-depth understanding of catalytic mechanism with the help of synchrotron source. XANES and EXAFS results revealed the surface oxidation occurred after electrocatalytic process. Besides, systematical characterizations such as HRTEM and EELS-mapping etc., further confirmed that thin NiOx layer formed in situ on the surface of Ni3C after electrochemical process, which was supposed to be the actual catalyst active site for oxygen evolution reaction. This work shed new light on catalytic mechanism for non-oxide metal catalysts as well as the design of highly efficient OER electrocatalysts. Article: Dr. K. Xu, H. Ding, P. Z. Chen, X. L. Lu, H. Cheng, T. P. Zhou, S. Liu, Prof. X. J. Wu, Prof. C. Z. Wu, Prof. Y. Xie. Dual Electrical-Behavior Regulation on Electrocatalysts Realizing Enhanced Electrochemical Water Oxidation. Adv. Mater. 2016, 28, 3326–3332. |
||||||
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility