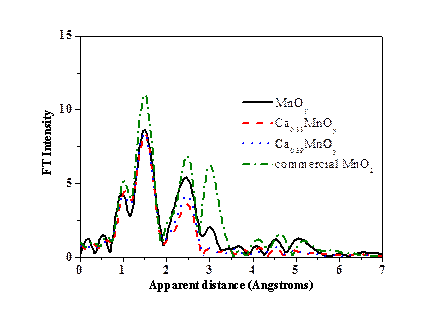| Highly Active Water Oxidation on Nanostructured Biomimetic Calcium Manganese Oxides Catalysts |
| From: PublishDate:2017-06-16 Hits: |
In natural photosynthesis, water oxidation reaction is catalyzed by μ-oxido-Mn4Ca cluster in photosystem II (PSII). It has been reported that the active site for natural PSII is CaMn4O5 cluster with three Mn and one Ca forming a distorted CaMn3O4 cubane and the fourth Mn ion bonding to this cubane by additional μ-oxido ligand. In order to reveal the mechanism of oxygen formation, synthesis of CaMn4O5 cluster mimetic is very important. A team from sate key laboratory of catalysis of Dalian institute of chemical physics has developed one-pot synthesis method for CaxMnOy nanoparticles and investigated water oxidation mechanism. Their research has been published on March 29th, 2016 in J. Mater. Chem. A. The team identified that CaxMnOy nanoparticles have similar structural motifs to the catalytic active site for water oxidation in PSII. Water oxidation experiments for both chemical and photocatalytic systems suggest that the disordered structure of calcium manganese oxides and a modest valence state of Mn (+3.7 ~ +3.8) are necessary for achieving high activity.
Using synchrotron radiation at BSRF, the local structure of CaxMnOy has been clarified. Calcium manganese oxides have similar structural motifs to the catalytic active site for water oxidation in PSII. The content of Ca and the concentration of H2O2 in initial mixture could affect the crystallinity and the average Mn valence state of calcium manganese oxides. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility