| Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction |
| From: PublishDate:2020-07-29 Hits: |
It is possible to efficiently convert CO2 into chemical products with certain economic value under mild conditions through electrochemical reduction, and at the same time realize the storage of clean electrical energy, showing great potential application prospects. Therefore, the development of an efficient CO2 electroreduction catalyst is a problem that needs to be solved urgently. Single-atom catalyst is a very popular direction in the field of catalysis and materials research in recent years. Since the active sites on the catalyst surface are highly dispersed, very high atom utilization rates can be achieved. At the same time, the active center interacts with the adjacent coordination atoms, causing the metal electronic structure of the active center to change. Therefore, in some catalytic reactions, such as CO oxidation reaction, hydrogenation reaction, oxygen reduction reaction, etc., can exhibit very excellent activity and selectivity. At present, there are still many difficulties in achieving large-scale production of single-atom catalysts through chemical synthesis, such as complex synthesis processes and harsh requirements on synthesis conditions. Therefore, the development of a simple and practical synthesis method that can produce monoatomic catalysts with thermal/chemical stability on a large scale is an urgent problem in the field of catalysis. In response to the above problems, Wu Yuen's research group of the University of Science and Technology of China, Li Yafei's research group of Nanjing Normal University and Duan Haohong's research group of Oxford University have developed a simple synthesis method. Through the solid-diffusion strategy, the bulk metal is directly converted into a hierarchical structure self-supported structure of single atom catalyst, this method is easy to achieve industrial-grade incremental preparation, and has broad industrialization prospects. This group found that melamine was evenly sprayed on the surface of the nickel foil, and the heating temperature was controlled under an argon atmosphere, so that the melamine was converted into a nitrogen-doped carbon layer. As the temperature increases, nickel continuously migrates out of the bulk nickel metal and is captured by the nitrogen-doped carbon layer. Due to the presence of some nickel particles in the nitrogen-doped carbon layer, at the high temperature of 1273K, a large amount of nitrogen-doped carbon tubes containing isolated nickel sites were formed on the surface of the nitrogen-doped carbon layer, and thus a hierarchical structure of carbon nanotubes/carbon layers/carbon nanotubes was formed. After cooling to room temperature, the paper-like catalyst film can be automatically detached from the surface of the nickel foil without any treatment, and the nickel foil can be reused after a simple polishing process. Scanning electron microscopy showed that the sample had a good carbon nanotubes/carbon layer/carbon nanotubes hierarchical structure. Transmission electron microscopy and aberration-corrected atomic microscopy showed that the surface nitrogen-doped carbon tube contained a large amount of isolated nickel sites. The presence of a nitrogen-doped carbon layer in the middle of the catalyst film gives the catalyst a certain degree of rigidity and flexibility, and can withstand bending with a certain force. The surface carbon tube further increases the surface area of the catalyst and gives a special surface wettability. This group can successfully prepare catalyst membranes of various shapes and even 50 cm2 in the laboratory by controlling the size of the nickel foil. This method can easily achieve large-scale preparation by expanding the nickel foil area. Since part of the nickel particles are well covered by the multi-layer graphene, the acid solution treatment cannot completely remove the nickel particles. However, the bare nickel particles on the surface can be removed by simple acid leached treatment, and the paper-like catalyst membrane after acid treatment can be directly used as a self-supporting electrode for CO2 electroreduction.
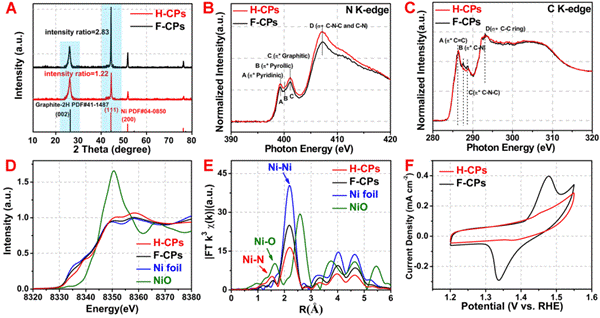
The EXAFS spectrum obtained by 1W1B-XAFS station at Beijing Synchrotron Radiation Facility (BSRF) suggest that the majority of Ni NPs could be removed after a chemical etching process in diluted HCl, leaving a small amount of Ni NPs encapsulated within the multiwall carbon nanotube. And further indicated that there was a large amount of Ni-N bonds in the sample, which further verified the existence of a large amount of isolated nickel sites in the catalyst At the same time, the NEXAFS spectrum showed that the carbon and nitrogen structure in the sample did not change significantly after acid treatment. Combined with the CV curve, it can be further explained that after the acid-dissolved process, the remaining nickel particles are well wrapped by the multi-layer graphene, so it is difficult to participate in the electrochemical reaction. The hierarchical catalyst membrane not only has certain rigidity and flexibility, so that it can be directly used as a self-supporting electrode. At the same time, the surface-rich carbon tubes increase the surface area of the catalyst while giving the catalyst film super-hydrophilic and super-aerophobic properties. In this paper, the water contact angle of the catalyst film is close to 0 °, while the contact angle of the bubbles is as high as 148.3 ± 2.6 °, showing excellent super-hydrophilic and super- aerophobic characteristics. Compared with the traditional method of dispersing the catalyst on carbon paper, the CO bubbles generated by the catalyst film with a hierarchical structure can be detached from the electrode surface more quickly, thus greatly improving the stability of the catalyst under high current density working conditions. The CO2 electroreduction conditions are closer to the actual industrial conditions. The super-hydrophilic and super-hydrophobic properties speed up the mass transfer of electroreduction. Compared with the traditional insulating polymer binders, (such as Nafion, PVDF), the catalyst is dispersed on the conductive substrate (such as carbon paper, glassy carbon electrode) and the current is further improved. In addition, the electrode is directly obtained during the catalyst synthesis process, without the need to re-disperse the catalyst, at the same time, without the need for adhesives and conductive substrate support, which greatly decrease costs and reduces the process flow. The easy large scale of preparation of the catalyst membrane also makes the catalyst more industrial potential. If you are interested in learning more about the details of synthesis and characterization, you can download the supplementary materials and videos of this article. This method of solid-phase thermal diffusion strategy has certain universality. The author successfully prepared a cobalt single-atom catalyst membrane with a hierarchical structure by the same method. This work reported for the first time that a solid-diffusion strategy can be used to directly prepare single-atom catalysts from bulk metal, and large-scale preparation of the catalyst can be achieved, recently published on Joule. This method provides new ideas for the design, preparation and scale-up of other highly efficient single-atom catalysts. Article: Changming Zhao, Yu Wang, Zhijun Li, Wenxing Chen, Qian Xu, Dongsheng He, Desheng Xi, Qinghua Zhang, Tongwei Yuan, Yunteng Qu, Jian Yang, Fangyao Zhou, Zhengkun Yang, Xiaoqian Wang, Jing Wang, Jun Luo, Yafei Li,* Haohong Duan,* Yuen Wu,* and Yadong Li. Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction. Joule, 2019, 3, 584-594. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility