| Efficient electrochemical reduction of CO2 |
| From: PublishDate:2020-07-30 Hits: |
Carbon dioxide (CO2) is the main greenhouse gas, and it is also a cheap, non-toxic, abundant and renewable C1 resource. Conversion of CO2 into high value-added chemicals has the dual meanings of rational utilization of carbon resources and environmental protection, and meets the requirements of sustainable development. Among all the technical routes, electrochemical reduction of CO2 to liquid fuel and high value-added chemicals is one of the most promising approaches with important application prospects. The key problem is to design efficient electrocatalytic systems to achieve efficient reduction of CO2 at low initial potential, low overpotential, and high current density. Buxing Han’s team from the Institute of Chemistry, Chinese Academy of Sciences has has gained insight into the research on the highly efficient electroreduction of CO2 and the related research results has been published in "Angewandte Chemie international Edition" and "Nature Communications". Electrochemical reduction of CO2 into CO is an attractive pathway. Han’s team reported to use atomic Ir electrocatalyst for CO2 reduction. By using α-Co(OH)2 as the support, the faradaic efficiency of CO could reach 97.6% with a turnover frequency (TOF) of 38290 h-1 in KHCO3 aqueous electrolyte, which is the highest TOF up to date. Interestingly, Ir nanoparticles (2 nm) had no catalytic activity under the same experimental conditions. The electrochemical active area was 23.4-times higher than Ir nanoparticles (2 nm), which is highly conductive and favors electron transfer from CO2 to its radical anion. Moreover, the more efficient stabilization of CO2 radical anion intermediate and easy charge transfer makes the atomic Ir electrocatalyst facilitate CO production.
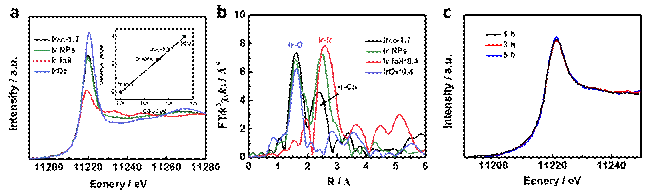
Fig. 1 a) Ir L3-edge XANES spectra of atomic Ir electrocatalyst. b) FT of the EXAFS spectra of IrAC-1.7. c) Ir L3-edge XANES spectra of IrAC-1.7 at -0.66 V versus RHE during CO2 reduction. XAS spectra carried out at 1W1B beamline of BSRF were used to study the electronic structure of atomic Ir electrocatalyst. According to X-ray absorption nearedge structure (XANES) spectra (Fig. 1a), the white line intensity at Ir L3-edge of IrAC-1.7 was between that of the standard reference IrO2 and Ir foil, suggesting the coexistence of Ir0 species and positively charged Ir species. The Fourier transform (FT) of the extended X-ray absorption fine structure (EXAFS) spectra for IrAC-1.7 in R space was obviously different from the reference samples and Ir NPs (Fig. 1b). Ir-O and Ir-Co coordination can be assigned in the spectra, and no Ir-Ir coordination, indicating atomic Ir was stabilized by surrounding O and Co sites of Co(OH)2. Besides, in situ XAS test was also performed to confirm the stablity of Ir electronic structure during CO2 reduction (Fig. 1c). This work provides a new approach to transform a material that is considered as nearly non-active for CO2 reduction into an active catalyst. The team also found that the copper complex-derived copper-copper oxide (Cu-Cu2O) catalyst can efficiently convert CO2 to C2 products. They used in situ electrodeposition method to fabricate Cu-complex film on Cu substrate, and in situ electroreduction of the Cu-complex film to obtain dendritic Cu-Cu2O/Cu electrode. The catalyst had outstanding performance for electrochemical reduction of CO2 to C2 products (acetic acid and ethanol). Under the applied potential of -0.4 V vs reversible hydrogen electrode, the overpotential was only 0.53 V (for acetic acid) and 0.48 V (for ethanol) with high C2 Faradaic efficiency of 80% and a current density of 11.5 mA cm-2. The Cu-complex precursor is the key to the construction of dendritic Cu-Cu2O structure. They used Small angle X-ray scattering (SAXS) technique carried out at 1W2A beamline of BSRF to analyze the fractal structure of the precursor, and studied the effect of the ligand on the roughness of the Cu-complex. Fig. 2 shows the lnI(h) vs. ln(h) plots of the complexes obtained from the SAXS data. It can be seen that Surface fractal (Ds) existed in the complexes, indicating that the surface of the complexes was coarse. The result indicates that the surface of the Cu-complex-1 film has a larger roughness, which is more conducive to the formation of dendritic Cu-Cu2O and increases the specific surface area of the electrode.
Fig. 2. lnI(h) vs. ln(h) plots of the complexes obtained from the SAXS data. In addition, they conducted in-depth research on the chemical states and bonding configurations of the catalysts. In this experiment, XAFS was applied to monitor the dynamic evolution of Cu species in the induction period of CO2 reduction (Fig. 3). From the XAFS result (Fig. 3a, b), it shows that a drastic shrink occurred to the CuI at 8981.0 eV and Cu0 peak at 8979.1eV in the first derivative curve after the reduction. In situ Cu K-edge XAFS spectroscopy results show that the energy of adsorption edge (E0) of Cu species increases with increasing oxidation degree. The E0 at 8979.1, 8980.9 and 8984.3 eV are characteristic of Cu foil, Cu2O and CuO reference electrodes respectively. It shows that with the increase of electrolysis time, the characteristic peaks of CuI and Cu0 exist on the electrode. The Cu K-edge extended XAFS oscillation function k3χ(k) and the corresponding Fourier transforms FT(k3χ(k)) results further revealed the existence of Cu-O and Cu-Cu in the catalyst after electroreduction. These results indicate that the Cu-complex gradually reduced to Cu-Cu2O during electrolysis, and the composition and morphology were stable after 5 minutes. As expected, the Cu-complex on the Cu substrate serves as a template to form Cu-Cu2O with a 3D dendritic structure. This mixed state is very beneficial for the electrochemical reduction of CO2. The outstanding performance of the electrode for producing the C2 products resulted mainly from near zero contacting resistance between the electrocatalysts and Cu substrate, abundant exposed active sites in the 3D dendritic structure and suitable Cu(I)/Cu(0) ratio of the electrocatalysts. This work will trigger many interesting work for the design of other new catalysts for the electroreduction of CO2 to C2 products.
Fig. 3. The fine structure change of Cu-Cu2O-1 electrocatalyst during CO2 reduction: The O L-edge (a) and Cu K-edge (b) XAFS curve for Cu-Cu2O-1 electrocatalysts during the CO2 reduction; Cu K-edge extended XAFS oscillation function k3χ(k) (c); the corresponding Fourier transforms FT(k3χ(k)) (d) for Cu-Cu2O-1 electrocatalysts during the CO2 reduction. Article: 1.Xiaofu Sun, Chunjun Chen, Shoujie Liu, Song Hong, Qinggong Zhu, Qingli Qian, Buxing Han, Jing Zhang, Lirong Zheng, Aqueous CO2 reduction with high efficiency using α-Co(OH)2-supported atomic Ir electrocatalysts, Angew. Chem. Int. Ed., 2019, 58, 4669-4673. 2.Qinggong Zhu, Xiaofu Sun, Dexin Yang, Jun Ma, Xinchen Kang, Lirong Zheng, Jing Zhang, Zhonghua Wu, Buxing Han, Carbon dioxide electroreduction to C2 products over copper-cuprous oxide derived from electrosynthesized copper complex, Nat. Commun. 2019, 10, 3851. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility