| Electronic coupling activated single-atomic Ru for enhanced OER |
| From: PublishDate:2020-07-30 Hits: |
Hydrogen, as a new energy source, has received much attention because of its high energy density and pollution-free. However, the development of hydrogen energy still faces huge challenges. The hydrogen production from water electrolysis is a simple process and the purity of the as-prepared hydrogen is relatively high. Water electrolysis includes two half reactions, namely, the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). Unfortunately, the kinetics of OER are rather sluggish due to the multiple proton-couple electron-transfer steps. Moreover, the commercial precious metal (Ru, Ir) based catalysts catalyzing OER have their problems, such as, the high price and poor stability, which severely limited the commercialization of electrolytic water. Monoatomic catalyst is alternative choice for solving the issue of electrolytic water catalysts due to the maximum atom utilization and the unique activity. However, using single-atom as a strategy to design electrocatalyst to overcome the issue of high cost and low stability of noble metal oxides like RuO2 is still challenge. Prof. Sun’ research group in Beijing University of Chemical Technology for the first time realized the preparation and detailed study of monoatomic Ru based catalyst efficient oxygen evolution reaction by virtue of the electronic coupling between CoFe-LDH and monoatomic ruthenium. The related results were published on April 12, 2019 in the Nature Communications journal: https://doi.org/10.1038/s41467-019-09666-0. The research team successfully prepared a supported monoatomic ruthenium catalyst by precipitation procedure using LDH containing transitional non-noble metals as the substrate. The boosting oxygen evolution reaction activity and durability were achieved by the strong electronic interaction between monoatomic ruthenium and LDH. The dispersed monoatomic ruthenium on the surface of CoFe-LDH not only reduces the cost but also exhibits higher performance.
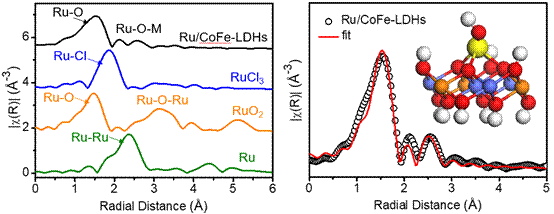
Using XAFS experiment carried out at 1W1B-XAFS station of Beijing Synchrotron Radiation Facility (BSRF), the authors confirmed that the monoatomic ruthenium was connected to the cobalt or iron atoms on the hydrotalcite layer through oxygen bridge bonds. The fitting results confirmed that the monoatomic ruthenium first shell oxygen coordination number is 3.9 ± 0.7. The synchrotron sources have helped the team to unveil the monoatomic structure. Based on the absorption energy edge of Ru, the authors confirmed the valence state of monoatomic ruthenium(+1.6), which is in a special chemical state.The authors say that the synchrotron radiation analysis provides a great help in confirming the state of monoatomic ruthenium. In the OER process, the overpotential at a current density of 10 mA / cm2 was only 198 mV with the Tafel slope of 39 mV dec-1. The performance was far superior to CoFe-LDH and commercial RuO2 catalysts. More importantly, the catalyst also showed excellent stability. At a given working voltage, the initial current density only decayed 1% after 24 hours of catalysis. This improvement in performance is mainly due to the electronic interaction between Ru and CoFe-LDH optimizing the electronic structure of the Ru active site. After subsequent in-situ test analysis, the authors found that Ru could be stabilized by CoFe-LDH in the valence state of less than +4 during OER process, which avoided the dissolution of Ru and maintained the catalyst's high activity. The new monoatomic Ru/CoFe-LDH catalyst has showed a great potential for the real application of water electrolysis in the future. Article: Pengsong Li, Maoyu Wang, Xinxuan Duan, Lirong Zheng, Xiaopeng Cheng, Yuefei Zhang, Yun Kuang, Yaping Li, Qing Ma, Zhenxing Feng*, Wen Liu*, and Xiaoming Sun*, Boosting oxygen evolution of single-atomic ruthenium through electronic coupling with cobalt-iron layered double hydroxides, Nature Communications , 2019, 10, 1711. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Science Highlights
Home /
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility