Electrocatalytic water splitting for oxygen generation is an multi-electron, multi-step process. The water adsorption and the formation of oxygen-producing intermediates are the main reason for the relative big overpotential. How to control the surface sites for promoting the water oxidation steps is one difficulty. A team from New Energy Research Institute of Shanxi Normal University use the W6+ for doing into α-Ni(OH)2 lattices as the signal-atom form. Their research has been published on May 14th, 2010 in Nature Communications.
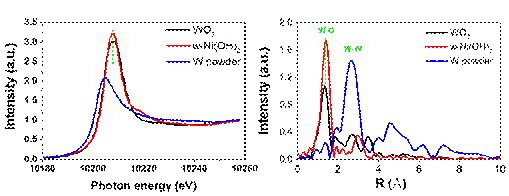
XAFS results carried out at 1W1B-XAFS station of BSRF. These results confirm that the W6+ exists as the form of W-O bonds in α-Ni(OH)2 and no W-W bonds can be detected, suggesting that the W6+ exists in the form of signal-atom doping.
The research provides the scientific community clues to control the surface reaction sites of Ni(OH)2-based materials for obtaining a high water oxidation performance. And the synchrotron sources have helped the team to confirm the existence form of element W, and provides a direct evidence for understand well about the water oxidation mechanism. "Signal-atom doping plays an important role in water oxidation owing to that the doped elements can provide the suitable reaction sites for water adsorption and the formation of intermediates." Explains Junqing Yan, the first author of this work and the associate professor of Shaanxi Normal university.
Article:
Junqing Yan, Lingqiao Kong, Yujin Ji, Jai White, Youyong Li, Jing Zhang, Pengfei An, Shengzhong Liu, Shuit-Tong Lee, and Tianyi Ma. Single atom tungsten doped ultrathin α-Ni(OH)2 for enhanced electrocatalytic water oxidation Nature Communications, 10(2019), 2149.


