| Water stable MOFs electrocatalysts for highly selective CO2 reduction |
| From: PublishDate:2020-07-31 Hits: |
Electrochemical reduction of CO2 can transform CO2 into useful chemicals and fuels by using renewable electric energy and water, which is an ideal energy utilization strategy. However, CO2 reduction generally need a high potential to derive and the side reactions of hydrogen evolution always occur concomitantly. It has become a critical issue to develop highly selective and active electrocatalysts. The CO2 reduction reaction involves proton and electron transfer processes, which play an important role for regulating the product selectivity and reaction kinetics. Hua Gui Yang’s Group of East China University of Science and Technology designed and synthesized a highly efficient and water stable MOFs electrocatalyst, and study the proton-electron transfer mechanisms in the reaction. Their research has been published on the 40th issue of Journal of Materials Chemistry A in 2019.
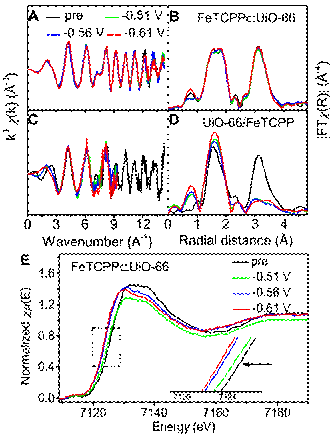
The researchers incorporated the iron porphyrin, possessing single active site for CO2 reduction, in to the frameworks of UiO-66, leading to an hydrostable MOFs electrocatalyst. The –OH groups on the surface of secondary building unit of UiO-66 could accelerate the proton transmission and the redox iron porphyrin improves the conductivity of UiO-66, leading a concerted proton-electron transfer. The accelerated proton transmission in the catalysts lowers the overpotential of reducing the CO2 to CO and enhances the product selectivity. Using XAFS technique carried out at 1W1B-XAFS station of BSRF, the local coordinated structural transformation and the active center of MOFs catalysts were demonstrated by the in-situ electrochemical characterization. As shown in Figures A-D, the local coordinated structure of Zr atoms in FeTCPP The research shows that FeTCPP Article: Fangxin Mao, Yan-Huan Jin, Peng Fei Liu, Pengfei Yang, Xiao-Ming Cao*, Jinlou Gou* and Hua Gui Yang*, Accelerated proton transmission in metal–organic frameworks for the efficient reduction of CO2 in aqueous solutions, Journal of Materials Chemistry A, 2019, 7 (40): 23055-23063. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility