| Study on phase transition and thermochromism of copper-based hybrid perovskites |
| From: PublishDate:2020-07-31 Hits: |
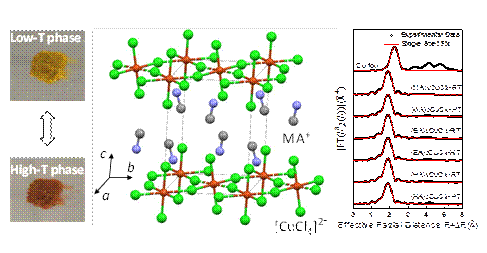
Organic-inorganic hybrid perovskites possess several appealing features such as tunable band gap, chemical versatility, and excellent optoelectronic property, and have recently attracted intense attention from the research community. Recently, it is reported that the thermochromic solar cells were fabricated based on halide perovskites (CsPbI3-xBrx). This breakthrough reveals a promising application of perovskite materials as the thermochromic layer in smart windows. The practical application, however, needs the hybrid perovskites to be stable, efficient, eco-friendly, and be used near room temperature. Researchers from Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences investigated the phase transition and thermochromism of copper-based perovskites, with the help of 1W1B-XAFS station at BSRF (Beijing Synchrotron Radiation Facility). They found a lead-free perovskite material (C6H13NH3)2CuCl4 that is thermochromic around 60℃. This work has been published in Science China Chemistry on July 11th, 2019. The organic cation (A) in the copper-based perovskite A2CuCl4 (A=CnH2n+1NH3 n=1-6) is found to be essential to the phase transition temperature of the material. As the number of carbon atoms increases, the thermochromic temperature first increases and then decreases. The thermochromism of copper-based perovskite originates from the change in the [CuCl4]2- geometry, while the organic cation can influence this structural change from the following two aspects. (i) The large organic cation introduces additional hindrance for [CuCl4]2- to accomplish the structural transition, as it needs to overcome the molecular motion of organic cation. (ii) The large organic cation has weak N-H···Cl hydrogen bonding with inorganic compositions that facilitates the transition.
The chemical environment of Cu2+ in copper-based perovskites (MA2CuCl4, EA2CuCl4 and HA2CuCl4, MA=CH3NH3, EA=C2H5NH3, HA=C6H13NH3) was investigated from the change of Cu-Cl bonds by using the temperature-dependent X-ray absorption fine structure (XAFS) measurement at BSRF. The results show that the coordination geometry is [CuCl4]2-, and the length of Cu-Cl bonds varies as the temperature increases, meaning that the chemical environment of Cu2+ changes during the phase transition. As a result, the ligand-to-metal charge transfer transition leads to a visible color change upon the shift of absorption spectral range. This study proves that the organic component in hybrid perovskites plays an important role in optimizing the optoelectronic performance, and are beneficial to the future development of optoelectronic functional materials. The phase transition of hybrid perovskites is determined by the change in coordination geometry around metal atom. It is usually difficult to characterize such a small change in coordination structure; however, the synchrotron light source equipped with temperature-controlled sample chamber enables us to analyze the structure of hybrid perovskite at both room temperature and high temperature. It provides direct experimental evidence for the phase transition and thermochromism of these materials. Article: Jingwen Li, Xiaolong Liu, Peixin Cui, Junmeng Li, Tao Ye, Xi Wang, Chuang Zhang,* and Yong Sheng Zhao* Lead-free thermochromic perovskites with tunable transition temperatures for smart window applications, Science China Chemistry 62(2019), 1257-1262. |
|
|
| Chinese
- Metal-free efficient photocatalyst for stable visible water splitting——Top ten major scientific progresses in China in 2015
- The nano-resolution imaging platform was awarded the first rate prize of Beijing Science and Technology in 2014
- Beamline 1W1 of BSRF started to runoperate in the couplingparasitic mode of BEPCII
- Synthesis of High Performance Polymer Materials for Field Effect-Transistors
- Surfactant molecular aggregates in green solvents
- GIXRD has played an important role in the characterization of organic thin-film transistors
Copyright © 2011 - 2012 Beijing Synchrotron Radiation Facility